Review of Alpha 7 Nachr Clinical Trials Schizophrenia
Introduction
Schizophrenia is a severe and enduring mental disorder and a worldwide health priority. It is the virtually prevalent psychotic disorder, with a global average incidence of xv.two per 100,000 inhabitants and a male:female ratio of i.4:1 (1). Schizophrenia is by far 1 of the leading causes of disability worldwide, generating a substantial economic brunt, with an annual estimated toll ranging from US$94 1000000 to United states of america$102 billion (ii). Clinically, schizophrenia is characterized by a heterogeneous range of symptoms classified under the following domains: positive symptoms (e.g., delusions and hallucinations), negative symptoms (e.1000., melancholia flattening, alogia, apathy, and social isolation), cognitive deficits (e.thou., speed of processing and attending deficits), and melancholia symptoms (e.g., hypothymia, mania, and anxiety) (3).
Almost antipsychotic agents have demonstrated efficacy in improving positive symptoms; yet, data on their efficacy in negative symptoms and cognitive deficits are limited (4–half-dozen). Cognitive deficits are considered one of the most disabling symptoms and are considered one of the best predictors of long-term functional disability (7–ten). Such recognition has been the main promoter for the development of new therapeutic approaches under the premise that improving cognitive impairments can indirectly result in improvements in patients' psychosocial functioning (11, 12). As a upshot, knowledge has get a priority treatment target.
The two chief approaches that have been developed are the psychosocial approach, which is based mainly on cognitive rehabilitation programs, and the pharmacological approach, which is based on the development of cognitive enhancers evaluated under the Measurement and Treatment Research to Amend Noesis in Schizophrenia (MATRICS) initiative.
Among the potential pharmacological therapeutic targets for developing cerebral enhancers, the MATRICS initiative has stressed the importance of the cholinergic organization (13). This pathway has been widely associated with the modulation of cognitive processes (eastward.g., attention and retentiveness) in healthy people. Donepezil and galantamine, which delay cognitive impairment in the before stages of Alzheimer's illness past acting on the cholinergic system (fourteen), were among the commencement cognitive enhancers studied in schizophrenia. Nevertheless, a contempo meta-analysis analyzing the efficacy of acetylcholinesterase inhibitors for cognitive deficits in schizophrenia has shown inconclusive results (15).
During the last decade, nicotinic acetylcholine receptors (nAChR) have been identified every bit a promising new therapeutic target. Specifically, the nicotinic subtype alpha-7 has been proposed as a potential target for the pharmacological treatment of neurocognitive impairment (sixteen). Although prove on the efficacy of nicotinic acetylcholine agonists is based on preclinical research (17), some preliminary clinical evidence has shown promising results. Postmortem studies have revealed reduced availability of α7 binding receptors (17) and reduced α7 expression in specific brain regions in schizophrenia, e.k., the hippocampus and frontal cortex (xviii). Studies addressing the presence of impairments in sensory gating written report a decreased ability to gate auditory and eye-tracking stimuli, primarily due to alteration of chromosome xv (18). Given that this chromosome contains the CHRNA7 gene, which encodes the α7 nAChR (18), such results seem to suggest the presence of an impaired cholinergic neurotransmission betoken (nineteen, 20). Diverse clinical studies have described the positive influence of nicotine in diverse cognitive domains of patients with schizophrenia, such every bit attending and working retentivity processes (21–23). Hence, pharmacological compounds targeting nicotinic receptors accept been considered potentially useful in the handling of these deficits. More specifically, pharmacological treatment targeting the α7 nAChR is equally a promising option that can raise cerebral processes and amend negative symptoms (24).
Contempo trials accept tried to show the procognitive event of new compounds targeting the α7 nAChR equally improver treatment to antipsychotic regimens, and in recent years, the several compounds identified as α7 nAChR agonists include RG3487, TC-5619, ABT-126, DMXB-A, and tropisetron. Despite the promising results for α7 nAChR agonists, prove for their efficacy as procognitive drugs remains inconclusive (25). This study aims to summarize evidence on the efficacy of α7 nAChR agonists vs. placebo when used as adjunctive treatment to ameliorate cognitive and negative symptoms in patients with schizophrenia.
Methods
Type of Studies
Articles were included if they fulfilled the post-obit conditions:
a) Inclusion of patients diagnosed with schizophrenia or schizophreniform, schizoaffective, delusional, or psychotic disorder non otherwise specified according to the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM) (DSM-III-R, DSM-4-R, or DSM-V) (3) or the International Classification of Mental Diseases (ICD) (ICD-nine or ICD-10) (26).
b) Randomized placebo-controlled or double-blind clinical trials, open up-label studies, or crossover studies. We excluded case series, observational designs, and studies with an north = ane design. We compared cognitive enhancers as improver treatments with add together-on placebo to detect possible effects. If an effect existed and was clinically relevant, we extended the comparing to include several cognitive enhancers as competitive treatments.
No language restrictions were imposed in the choice of articles.
Participants and Interventions
We included trials with patients diagnosed with schizophrenia or a schizophreniform, schizoaffective, delusional, or psychotic disorder not otherwise specified, co-ordinate to DSM (3) or ICD (26) criteria. Patients had to be clinically stable and treated with typical or atypical antipsychotic agents. Experimental interventions were α7 nAChR as adjunctive medication to antipsychotic treatment. We included α7 nAChR agonists irrespective of the elapsing of treatment and the doses. The comparator intervention was matching placebo as adjunctive medication to antipsychotic handling.
Outcomes
Primary Outcome—Cognitive Domains
We assessed efficacy according to the results of cognitive tests and batteries based on the main cerebral domains afflicted in schizophrenia proposed by the MATRICS initiative: attending/vigilance, speed of processing, working memory, exact learning, visual learning, problem solving, and social cognition (vii). For each domain, we selected those psychometric measures that were replicated across trials or considered representative of a specific domain. For this purpose, we used the systematic review of Bakkour et al. (27) and the monograph of Lezak et al. (28) every bit general guidelines to identify and categorize the main cognitive domains evaluated through each cognitive exam. Table 1 illustrates the principal cognitive tests and batteries included clustered under the main domains proposed by the MATRICS initiative (seven).
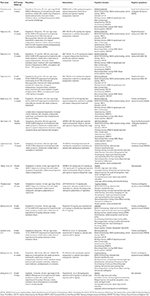
Table one. Characteristics of the studies included in the systematic review and meta-analysis of cognitive deficits and negative symptoms in schizophrenia.
Secondary Outcome—Negative Symptoms
For the assessment of negative symptoms, we considered those studies that used standardized instruments to evaluate negative clinical symptoms such as the Negative Symptoms Assessment Calibration (NSA-16) (42), the Positive and Negative Syndrome Scale for Schizophrenia (PANSS) (43), and the Scale for Negative Symptoms Assessment (SANS) (Tabular array one) (44).
Search Methods for the Identification of Studies
A search strategy was developed to identify potential studies (see search strategy in Supplementary Appendix 1). We adult a search strategy for MEDLINE (Ovid), Embase (Ovid), and the Cochrane Key Register of Controlled Trials (CENTRAL) and searched for RCTs upwardly to May 2019. We performed the search without language or date restrictions. The references of potentially eligible trials and relevant reviews were searched for additional citations.
Data Extraction and Analysis
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement to design the search flowchart. After removing duplicate records, two independent and blinded review authors (JB, MR) screened the titles and abstracts of the studies obtained from the literature searches to appraise eligibility. We obtained full reports from the studies considered to be potentially eligible. Disagreements were resolved by give-and-take and consensus.
The data extracted included the post-obit: primary and secondary outcomes, number of patients included, population characteristics (including historic period, sex, elapsing of affliction, and diagnosis), smoking condition, duration of treatment, consequence scales used, and tests for cognitive and negative assessment (i.e., mean, standard deviation). In instance of missing event data, the corresponding author of the written report was contacted via electronic mail.
The study characteristics and quantitative data were extracted using a information extraction sheet course. Sample sizes, means, and standard deviations from each intervention were obtained for each relevant event to calculate standardized mean differences (SMDs). We applied SMDs owing to the different measures, clinical tests, and metrics used to appraise cognitive deficits and negative symptoms. Nosotros interpreted SMDs as pocket-sized effect, medium issue, or large effect according to Cohen's criteria, with 0.2 representing a small event, 0.5 a moderate outcome, and 0.8 a large effect (45).
Afterwards a grooming practise, two contained authors (JB, MR) assessed the risk of bias for each trial included to determine study quality according to the PRISMA guidelines and the Cochrane Handbook of Systematic Reviews of Interventions (46). Run a risk of bias was classified every bit depression, unclear, or high. Disagreements were resolved past discussion and consensus. Nosotros used a random-effects model to combine individual effect sizes (47).
We used the I 2 statistic to appraise the heterogeneity of the studies. The I 2 statistic quantifies inconsistencies betwixt the studies. We considered I 2-values >60% as indicating substantial heterogeneity and conducted a subgroup analysis if viable to explain possible sources of heterogeneity. All analyses were performed using R (https://cran.r-project-org/), specifically the packages "meta" and "metaphor" (48, 49).
Finally, we assessed reporting bias depending on the availability of the study protocols described in the methodology section of each trial. The overall quality of bear witness for each cognitive and negative measure was presented according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach (50). Nosotros judged the quality of evidence considering the following dimensions: (a) study limitations (risk of bias across the studies at the outcome level), (b) inconsistency, (c) imprecision, (d) indirectness, and (due east) publication bias. Two review authors (JB, MR) independently rated the quality of bear witness for each outcome every bit high, moderate, low, or very low according to the GRADE framework (fifty). To generate an evidence table for each comparison, we used the online Guideline Development Tool (https://gradepro.org).
Results
Characteristics of the Studies Included and Patients
Our search identified 393 manufactures. After excluding duplicates, 254 references were reviewed past championship and abstruse, and 15 studies were considered eligible and assessed as full text; of these, thirteen studies were included in the nowadays review. Four studies were included in the quantitative assay for cognitive outcomes and nine studies were included in the quantitative assay for negative symptoms (Figure 1).
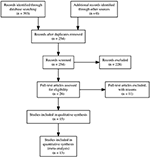
Effigy 1. Preferred Reporting Items for Systematic Reviews and Meta-assay (PRISMA) flow diagram.
Thirteen studies compared the effectiveness of antipsychotics plus α7 nAChR agonists with antipsychotics plus placebo for treatment of cognitive deficits. Of these, three studies used ABT-126 (30–32) or DMXB-A (29, 34, 36), two studies used TC-5619 (35, 40) or tropisetron (38, 41), and one study used encenicline (33), EVP-6124 (37), or RG 3487 (39).
To appraise the effectiveness of antipsychotics plus α7 nAChR agonists vs. placebo in negative symptoms, nosotros finally included for analysis three studies using ABT-126 (xxx–32), two studies using tropisetron (38, 51) or DMXB-A (29, 34), and 1 report using RG 3487 (39) or citicoline (52) (Table 1). The sample size of the studies ranged from 12 to 477 participants, with a median of 153 participants (IQR 40 to 215).
Risk of Bias of the Studies Included in the Qualitative Synthesis
The overall risk of bias across the studies is presented in Figure 2. Except for two studies (29, 41), all studies were reported as randomized and provided appropriate information to assess the randomization sequence procedure. Overall, the risk of bias of random sequence generation was rated as depression. Five studies (29, 33, 39, 41, 52) presented an unclear adventure for allocation darkening. Overall, the risk of bias of allocation concealment was rated equally unclear. All studies reported appropriate information for blinding weather condition regarding participants and personnel and upshot assessment. The hazard of bias for blinding participants, personnel, and assessors was rated as low for all outcomes. 1 study presented an unclear risk for incomplete information outcomes (29), since it did not provide data on loss or withdrawal of patients. The report by Haig et al. (31) was rated as having a loftier hazard of bias, since when losses in treatment were described, differences were observed between the handling artillery or betwixt the interventions. Overall, the risk of bias for incomplete outcome information was rated low for all outcomes. As for selective reporting, all the studies reported on clinical trial registration. However, one report (33) was reported equally having an unclear take chances for selective reporting due to chief and secondary outcome-specific variables that were non provided in the clinical trial protocol. We also considered that Freedman et al. (29) presented a loftier risk of bias because the variables and measures reported in the registered clinical trial protocol did non coincide with those presented in full-text articles. Finally, except for the studies carried out past Zhang et al. (41) and Nozoorian et al. (51), all the other studies included in the qualitative assay presented a potent suspicion of publication bias owing to the direct participation of the pharmaceutical industry.

Figure 2. Risk of bias summary of included studies.
Effects of Interventions
A summary of the main findings is presented in Figure three.
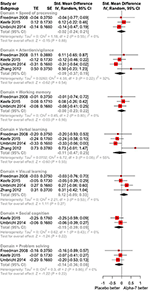
Effigy 3. Overall efficacy by cognitive domain of antipsychotic drug plus α-7 nAChR agonists vs. antipsychotic drug plus placebo.
Primary Outcome—Cognitive Deficits
Speed of Processing
Based on all available testify (three studies, including 376 randomized participants: 268 on α7 nAChR agonists, 108 on placebo), and given the depression quality of testify, adding α7 agonists to antipsychotic treatment has little or no effect on speed of processing. SMDs are compatible with pocket-size to moderate effects, supporting either α7 agonists or placebo (SMD −0.02, 95% CI −0.24 to 0.xx; P = 0.86) (Figure 3). Effect estimates were not statistically heterogeneous (χ2 = i.22 on 2 df, P = 0.54, I 2 = 0%). Nosotros downgraded the quality of evidence owing to a potent suspicion of publication bias.
Attention
Based on three studies including 413 randomized participants (296 on α7 nAChR agonists, 117 on placebo) and providing low-quality bear witness, calculation α7 agonists to antipsychotic treatment may have little or no effect on attention. SMD values showed a compatible small to moderate issue supporting either α7 agonists or placebo (SMD −0.09, 95% CI −0.37 to 0.02, P = 0.56) (Figure 3). Heterogeneity in effect estimates was depression (χii = iv.51 on three df, P = 0.21, I two = 34%). We downgraded the quality of evidence owing to a strong suspicion of publication bias.
Working Memory
Based on three studies, including 380 randomized participants (268 on α7 nAChR agonists, 112 on placebo) and providing low-quality prove, adding α7 agonists to antipsychotic treatment may have little or no outcome on working memory. The SMD showed a modest to moderate outcome supporting either α7 agonists or placebo (SMD 0.00, 95% CI −0.23 to 0.22, P = 0.97) (Figure iii). Effect estimates were not statistically heterogeneous (χtwo = 0.42 on two df, P = 0.81, I ii = 0%). We downgraded the quality of prove owing to a stiff suspicion of publication bias.
Verbal Learning
Based on four studies including 414 randomized participants (296 on α7 nAChR agonists, 118 on placebo) and providing depression-quality of evidence, adding α7 agonists antipsychotic treatment had little or no upshot on verbal learning. SMD values are compatible with a small to moderate event supporting α7 agonists and moderate effects supporting placebo (SMD −0.11, 95% CI −0.47 to 0.25, P = 0.55) (Effigy 3). Issue estimates were moderately heterogeneous (χ2 = six.72 on iii df, P = 0.08, I 2 = 55%). We downgraded the quality of show owing to a strong suspicion of publication bias.
Visual Learning
Based on 4 studies, including 414 randomized participants (296 on α7 nAChR agonists, 118 on placebo) and providing depression-quality bear witness, adding α7 agonists to antipsychotic treatment may accept little or no consequence on visual learning. SMD values are compatible with a small-scale to moderate effect supporting α7 agonists and a very small effect supporting placebo (SMD 0.12, 95% CI −0.09 to 0.34, P = 0.26) (Figure iii). Upshot estimates were not statistically heterogeneous (χ2 = 2.22 on iii df, P = 0.53, I ii = 0%). We downgraded the quality of testify owing to a strong suspicion of publication bias.
Problem Solving
Based on three studies, including 376 randomized participants (268 on α7 nAChR agonists, 108 on placebo) and providing low-quality evidence, adding α7 agonists to antipsychotic treatment may take picayune or no effect on reasoning/problem solving. The SMD values are uniform with a very pocket-size effect supporting α7 agonists and a moderate effect supporting placebo (SMD −0.14, 95% CI −0.36 to 0.08, P = 0.22) (Figure 3). Effect estimates were not statistically heterogeneous (χtwo = 0.28 on 2 df, P = 0.87, I 2 = 0%). Nosotros downgraded the quality of evidence attributable to a strong suspicion of publication bias.
Social Cognition
Based on ii studies including 345 randomized participants (248 on α7 nAChR agonists, 97 on placebo) and providing low-quality evidence, α7 agonists have little or no upshot on problem solving. The SMD values are compatible with a minimal effect supporting α7 agonists and a moderate effect supporting placebo (SMD −0.15, 95% CI −0.39 to 0.08, P = 0.xx) (Figure 3). Effect estimates were not statistically heterogeneous (χtwo = 0.62 on 1 df, P = 0.43, I 2 = 0%). We downgraded the quality of evidence owing to a strong suspicion of publication bias.
Secondary Event—Negative Symptoms
Based on nine studies including 978 randomized participants (600 on α7 nAChR agonists, 378 on placebo) and providing low-quality evidence, calculation α7 agonists to antipsychotic treatment may accept a low to moderate effect on negative symptoms. The SMD values are compatible with a moderate result supporting α7 agonists over placebo (SMD −0.28, 95% CI −0.56 to −0.00, P = 0.05) (Figure four). Effect estimates were statistically heterogeneous (χtwo = 29.79 on 8 df, P < 0.001, I 2 = 73%). Because such heterogeneity, we observed that the study of Noroozian et al. (51) had a major impact on overall heterogeneity and effect size. Its removal from the show ready decreased I ii from 73 to 55% and the SMD to −0.fifteen (95% CI −0.39, 0.08). Whereas, heterogeneity between furnishings beyond studies decreased, the SMD as well decreased from −0.28 to −0.xv. We downgraded the quality of show owing to a strong suspicion of publication bias and unexplained heterogeneity (inconsistency).
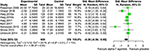
Figure iv. Overall efficacy in negative symptomatology of antipsychotic drug plus α-7 nAChR agonists vs. antipsychotic drug plus placebo.
Give-and-take
This systematic review and meta-analysis examined the efficacy of adding α7 nAChR agonists to antipsychotic treatment in cerebral deficits and negative symptoms in patients diagnosed with a schizophrenia spectrum disorder. Overall, α7 agonists may be more efficacious than placebo, even though the quality of prove remains low attributable to incertitude over the quality of evidence.
Our chief finding was that at that place was no general outcome of α7 nAChR agonists equally adjunctive treatment on cognitive deficits. Our results back up the view that calculation α7 nAChR agonists to habitual antipsychotic medication has little or no outcome on any of the cognitive domains evaluated. We downgraded the quality of bear witness to depression quality in all of the cerebral domains assessed attributable to suspected publication bias. Consistent with our results, a 2017 systematic review and meta-analysis (53) analyzing the effect of α7 and α4β2 receptor agonists in patients with schizophrenia did not reveal an overall improvement in cognitive (or negative) outcomes when measuring composite cognitive scores. Moreover, in this study, the individual cerebral domains typically affected in these patients were not taken into consideration separately.
Nosotros did non evaluate the outcome of α7 nAChR agonists on overall cognitive function, equally nosotros do not consider that a composite neurocognitive mensurate is a valid representative measure for the evaluation of the real cognitive effects of a specific handling. Thus, nosotros avoided this problem by evaluating the main cognitive domains proposed by the MATRICS initiative (seven) to estimate the effect of these treatments on cognitive performance. Our view is that the assessment of private cognitive domains is essential for estimating the actual upshot of those treatments on the cognitive areas that are prototypically affected in schizophrenia. Besides, this approach is of item importance in the social noesis domain, as the outcome observed has been shown to be strongly correlated with the daily operation of schizophrenia patients (54, 55).
There was no evidence of heterogeneity in whatsoever specific cognitive domain (I two < lx%). As mentioned previously, evidence of reporting bias was suspected for all cognitive domains, owing to partial presentation of raw data for specific cognitive domains. Reporting of information in both full-text format and equally supplemental textile was deficient in well-nigh studies, except the four studies included in the quantitative assay (29, 33, 39, 41).
Regarding negative symptoms, α7 nAChR agonists do present moderate effects equally adjunctive treatment on negative symptoms. Although there was considerable heterogeneity among the studies reporting negative symptoms (I 2 = 73%), this state of affairs does not seem to be accounted for by other clinical moderators. For that reason, we did not perform a subgroup analysis. We did indeed influence analyses past removing one report at a time from the evidence prepare. As presented in the results section, the residue heterogeneity observed afterwards removing the study of Noroozian et al. (51) could exist explained using a random-effects analysis model, in which it is considered that the studies included are a random sample of the population of all studies and are weighted. Finally, we downgraded the quality of evidence because of a strong suspicion of publication bias with respect to negative symptoms.
Only four of 13 studies complied with the Consort guidelines for reporting clinical trials and presented raw data to assess the efficacy of α7 nAChR agonists on cognitive deficits. It is remarkable that fifty-fifty though cognition was considered the master measure in most of the studies included, the completeness of data was greater for clinical outcomes, with a total of ix trials providing either full-text data or Supplementary Material for the assessment of negative symptoms. Transparency and abyss in reporting biomedical research are essential when evaluating the methodological quality and reproducibility of clinical trials. Despite the development of the Espoused statement in 2010 to provide an evidence-based checklist of recommendations for reporting the findings of randomized clinical trials, a big proportion of biomedical trials do not yet provide sufficiently high-quality reporting.
Nearly of the clinical trials included in this review did non report a duration of handling beyond 12 weeks, except for that of Walling et al. (xl), where follow-upwardly was longer than the average and information were analyzed at weeks 12 and 24. Moreover, and from a clinical betoken of view, it is interesting to consider the potential outcome of these prognostic drugs on patients' day-to-day functionality. While evidence has shown that improvements in cognitive performance correlate with actual patient performance, recommendations from institutions, such every bit the FDA-NIMH, take highlighted the need to include variables that assess the impact on patient functionality as co-chief measures (56). Despite this, only 6 studies considered the inclusion of measures to assess patients' functional capacity.
Information technology is also necessary to consider that patients included in nearly clinical trials may non exist representative of daily clinical do, as inclusion criteria are oftentimes very restrictive (e.g., chronically sick patients with stable symptoms, college rates of adherence, or patients non presenting another psychiatric comorbidity, such as substance abuse or dependence). Implementation of such strategies in daily clinical practice could be limited. Overall, the quality of prove is low, suggesting that prescribing α7 nAChR agonists as adjunctive therapy does not seem to amend the prototypical clinical deficits of schizophrenia, such as negative and cognitive symptoms.
Limitations
Our work is subject to a series of limitations.
First, we could not include some studies because the authors did not provide enough information, either as total text or Supplementary Material. In addition, almost studies did not present appropriate data in the methods department to assess the run a risk of bias in the study and outcomes. None of the authors we contacted to obtain supplementary information and information for cerebral and negative measures responded to our requests. For this reason, the quantitative analysis for cognitive outcomes includes a very small-scale number of studies.
The large number of cerebral tasks and batteries widely used in the literature to evaluate cerebral deficits in patients with schizophrenia fabricated assessment of the intervention effects of a specific treatment over the years challenging. Additionally, while studies frequently report combined or pooled cognitive measures as a standard general measure out of real cerebral functioning, composite measures are difficult to understand and interpret. Given this heterogeneity, the MATRICS initiative emerged to provide unified reporting criteria for the primary cognitive processes afflicted in schizophrenia (vii). Even if the MATRICS initiative is bailiwick to methodological and clinical criticisms, it has made it possible to frame cognitive constructs and select standardized cognitive measures to assess cerebral functions.
The boilerplate duration of clinical trials is short. In general terms, clinical trials evaluating the effectiveness of cognitive enhancers in schizophrenia are shorter than 12 weeks. As stated by Dark-green et al. (54), the desirable effects on cognition, and even more so on the patient'due south functioning, can exist expected over a period of half-dozen to 12 months. Equally a result, the studies included may non take been sufficiently long to provide a genuine cess of the long-term efficacy and safety of cognitive enhancers (54).
Since a wide range of cognitive alterations are present prior to the onset of the disease and these follow a stable course (57, 58), information technology is necessary to consider the inclusion of patients with a first psychotic episode. The inclusion of this group could help united states of america to understand and evaluate those treatments in more recently diagnosed patients and, with it, prevent resistance to pharmacological treatment.
Conclusions
To summarize, we found no evidence of the effectiveness of α7 nAChR as add together-on treatment for cognitive deficits in schizophrenia. Although there is a small effect supporting the use of α7 nAChR agonists for negative symptoms, the general quality of show is depression. To provide a more accurate picture of the actual furnishings of such treatments, nosotros demand adequately powered clinical trials, as well as a preregistration and/or clear reporting of methods and outcomes in accessible protocols, in club to foreclose both reporting bias and publication bias.
Data Availability Argument
The original contributions presented in the written report are included in the article/Supplementary Cloth, further inquiries can be directed to the respective writer/due south.
Author Contributions
MR-B, RS, AZ, EG-F, AG-P, and JB contributed to the blueprint, development, and final typhoon of the protocol. EG-F, MR-B, and JB conducted the systematic literature search. RS and MR-B fabricated the trial choice and obtained full-text reports. RS and AZ extracted the primary written report characteristics. MR-B and JB extracted effect sizes from the studies included, assessed the risk of bias, and assessed the quality of prove (Class). JB led the information analysis. AZ, RS, AG-P, and MR-B assisted with information interpretation. All review authors contributed to the final typhoon.
Funding
The work conducted at the University of the Basque State was funded by public grants GIU14/27, PPGA18/03, and IT1232-nineteen.
Conflict of Interest
The authors declare that the research was conducted in the absenteeism of whatever commercial or financial relationships that could be construed as a potential conflict of involvement.
Supplementary Material
The Supplementary Material for this article tin be found online at: https://www.frontiersin.org/articles/x.3389/fpsyt.2021.631589/full#supplementary-material
References
2. Chong HY, Teoh SL, Wu DB, Kotirum S, Chiou CF, Chaiyakunapruk North. Global economic brunt of schizophrenia: a systematic review. Neuropsychiatr Dis Treat. (2016) 12:357–73. doi: 10.2147/NDT.S96649
PubMed Abstract | CrossRef Total Text | Google Scholar
three. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th Ed.). Arlington, VA: American Psychiatric Association, (2013). doi: 10.1176/appi.books.9780890425596
CrossRef Total Text | Google Scholar
5. Citrome Fifty. A systematic review of meta-analyses of the efficacy of oral atypical antipsychotics for the handling of developed patients with schizophrenia. Expert Opin Pharmacother. (2012) 13:1545–73. doi: 10.1517/14656566.2011.626769
PubMed Abstract | CrossRef Full Text | Google Scholar
six. Fusar-Poli P, Papanastasiou Due east, Stahl D, Rocchetti M, Carpenter W, Shergill S, et al. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomised placebo-controlled trials. Schizophr Bull. (2015) 41:892–9. doi: 10.1093/schbul/sbu170
PubMed Abstract | CrossRef Full Text | Google Scholar
7. Greenish MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional issue in schizophrenia: implications for MATRICS. Schizophr Res. (2004) 72:41–51. doi: ten.1016/j.schres.2004.09.009
PubMed Abstract | CrossRef Total Text | Google Scholar
9. Harvey PD, Stressing Thou. Predicting the severity of everyday functional disability in people with schizophrenia: cognitive deficits, functional capacity, symptoms, and wellness status. World Psychiatry. (2012) 11:73–9. doi: x.1016/j.wpsyc.2012.05.004
PubMed Abstract | CrossRef Full Text | Google Scholar
x. Hang WC, Hui CL, Chan SK, Lee EH, Chen EY. Bear upon of avolition and cognitive harm on functional outcome in first-episode schizophrenia-spectrum disorder: a prospective one-year follow-upwards study. Schizophr Res. (2016) 170:318–21. doi: 10.1016/j.schres.2016.01.004
PubMed Abstruse | CrossRef Total Text | Google Scholar
11. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional upshot in schizophrenia: are we measuring the "right stuff"? Schizophr Bull. (2000) 26:119–36. doi: ten.1093/oxfordjournals.schbul.a033430
PubMed Abstract | CrossRef Total Text | Google Scholar
12. Kuipers E, Yesufu-Udechuku A, Taylor C, Kendall T. Management of psychosis and schizophrenia in adults: summary of updated Dainty guidance. BMJ. (2014) 348:g1173. doi: 10.1136/bmj.g1173
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Birks J. Cholinesterase inhibitors for Alzheimer's disease. Cochrane Database Syst Rev. (2006) 25:CD005593. doi: ten.1002/14651858.CD005593
CrossRef Full Text | Google Scholar
15. Santos B, González-Fraile E, Zabala A, Guillén V, Rueda JR, Ballesteros J. Cognitive comeback of acetylcholinesterase inhibitors in schizophrenia. J Psychopharmacol. (2018) 32:1155–66. doi: 10.1177/0269881118805496
PubMed Abstract | CrossRef Total Text | Google Scholar
17. Lewis AS, van Schalkwyk GI, Bloch MH. Alpha-7 nicotinic agonists for cerebral deficits in neuropsychiatric disorders: a translational meta-assay of rodent and man studies. Prog Neuropsychopharmacol Biol Psychiatry. (2017) 75:45–53. doi: 10.1016/j.pnpbp.2017.01.001
PubMed Abstract | CrossRef Full Text | Google Scholar
xviii. Rowe AR, Mercer L, Casetti V, Sendt KV, Giaroli One thousand, Shergill SS, et al. Dementia praecox redux: a systematic review of the nicotinic receptor as a target for cognitive symptoms of schizophrenia. J Psychopharmacol. (2015) 29:197–211. doi: 10.1177/0269881114564096
PubMed Abstract | CrossRef Total Text | Google Scholar
19. Adler LE, Olincy A, Waldo M, Harris JG, Griffith J, Stevens K, et al. Schizophrenia, sensory gating, and nicotinic receptors. Schizophr Balderdash. (1998) 24:189–202. doi: ten.1093/oxfordjournals.schbul.a033320
PubMed Abstract | CrossRef Full Text | Google Scholar
21. Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, et al. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology. (2004) 29:1378–85. doi: 10.1038/sj.npp.1300450
PubMed Abstruse | CrossRef Full Text | Google Scholar
22. Segarra R, Zabala A, Eguíluz JI, Ojeda Northward, Elizagarate E, Sánchez P, et al. Cerebral performance and smoking in first-episode psychosis: the self-medication hypothesis. Eur Arch Psychiatry Clin Neurosci. (2011) 261:241–50. doi: 10.1007/s00406-010-0146-6
PubMed Abstruse | CrossRef Total Text | Google Scholar
23. Zabala A, Eguiluz JI, Segarra R, Enjuto S, Ezcurra J, González Pinto A, et al. Cognitive performance and cigarette smoking in first-episode psychosis. Eur Arch Psychiatry Clin Neurosci. (2009) 259:65–71. doi: 10.1007/s00406-008-0835-half dozen
PubMed Abstract | CrossRef Full Text | Google Scholar
25. Bristow LJ, Easton AE, Li YW, Sivarao DV, Lidge R, Jones KM, et al. The novel, nicotinic alpha7 receptor partial agonist, BMS-933043, improves cognition and sensory processing in preclinical models of schizophrenia. PLoS I. (2016) 11:e0159996. doi: x.1371/journal.pone.0159996
PubMed Abstract | CrossRef Total Text | Google Scholar
26. The ICD-10 Nomenclature of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization (1992).
Google Scholar
27. Bakkour N, Samp J, Akhras K, El Hammi E, Soussi I, Zahra F, et al. Systematic review of appropriate cognitive cess instruments used in clinical trials of schizophrenia, major depressive disorder and bipolar disorder. Psychiatry Res. (2014) 216:291–302. doi: x.1016/j.psychres.2014.02.014
PubMed Abstract | CrossRef Total Text | Google Scholar
28. Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. New York, NY: Oxford University Printing. (2012)
Google Scholar
29. Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. (2008) 165:1040–7. doi: ten.1176/appi.ajp.2008.07071135
PubMed Abstruse | CrossRef Full Text | Google Scholar
30. Haig GM, Bain EE, Robinson WZ, Bakery JD, Othman AA. A randomised trial to appraise the efficacy and rubber of ABT-126, a selective α7 nicotinic acetylcholine receptor agonist, in the treatment of cerebral damage in schizophrenia. Am J Psychiatry. (2016) 173:827–35. doi: 10.1176/appi.ajp.2015.15010093
PubMed Abstract | CrossRef Full Text | Google Scholar
31. Haig One thousand, Wang D, Othman AA, Zhao J. The α7 nicotinic agonist ABT-126 in the treatment of cognitive impairment associated with schizophrenia in nonsmokers: results from a randomized controlled phase 2b study. Neuropsychopharmacology. (2016) 41:2893–902. doi: 10.1038/npp.2016.101
PubMed Abstract | CrossRef Full Text | Google Scholar
32. Haig GM, Wang D, Zhao J, Othman AA, Bain EE. Efficacy and safety of the α7-Nicotinic acetylcholine receptor agonist ABT-126 in the handling of cerebral harm associated with schizophrenia: results from a phase 2b randomized controlled study in smokers. J Clin Psychiatry. (2018) 79:16m11162. doi: 10.4088/JCP.16m11162
PubMed Abstract | CrossRef Full Text | Google Scholar
33. Keefe RS, Meltzer HA, Dgetluck Due north, Gawryl M, Koenig G, Moebius HJ, et al. Randomized, double-blind, placebo-controlled report of encenicline, an α7 nicotinic acetylcholine receptor agonist, as a handling for cognitive impairment in schizophrenia. Neuropsychopharmacology. (2015) 40:3053–sixty. doi: x.1038/npp.2015.176
PubMed Abstract | CrossRef Total Text | Google Scholar
34. Kem WR, Olincy A, Johnson 50, Harris J, Wagner BD, Buchanan RW, et al. Pharmacokinetic limitations on furnishings of an Alpha7-Nicotinic receptor agonist in schizophrenia: randomised trial with an extended-release formulation. Neuropsychopharmacology. (2018) 43:583–nine. doi: 10.1038/npp.2017.182
PubMed Abstract | CrossRef Full Text | Google Scholar
35. Lieberman JA, Dunbar M, Segreti AC, Girgis RR, Seoane F, Beaver JS, et al. A randomised exploratory trial of an alpha-7 nicotinic receptor agonist (TC-5619) for cognitive enhancement in schizophrenia. Neuropsychopharmacology. (2013) 38:968–75. doi: 10.1038/npp.2012.259
PubMed Abstruse | CrossRef Full Text | Google Scholar
36. Olincy A, Harris JG, Johnson LL, Pender Five, Kongs S, Allensworth D, et al. Proof-of-concept trial of an α7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. (2006) 63:630–8. doi: 10.1001/archpsyc.63.6.630
PubMed Abstract | CrossRef Full Text | Google Scholar
37. Preskorn SH, Gawryl Thou, Dgetluck Northward, Palfreyman Thousand, Bauer LO, Hilt DC. Normalising effects of EVP-6124, an α-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomised trial in patients with schizophrenia. J Psychiatr Pract. (2014) xx:12–24. doi: 10.1097/01.pra.0000442935.15833.c5
PubMed Abstruse | CrossRef Full Text | Google Scholar
38. Shiina A, Shirayama Y, Niitsu T, Hashimoto Y, Yoshida T, Hasegawa T, et al. A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann Gen Psychiatry. (2010) 9:27. doi: 10.1186/1744-859X-nine-27
PubMed Abstract | CrossRef Full Text | Google Scholar
39. Umbricht D, Keefe RS, Murray S, Lowe DA, Porter R, Garibaldi G, et al. A randomised, placebo-controlled written report investigating the nicotinic α 7 Agonist, RG3487, for cognitive deficits in schizophrenia. Neuropsychopharmacology. (2014) 39:1568–77. doi: 10.1038/npp.2014.17
PubMed Abstruse | CrossRef Full Text | Google Scholar
40. Walling D, Marder SR, Kane J, Fleischhacker WW, Keefe RS, Hosford DA, et al. Phase 2 trial of an alpha-7 nicotinic receptor agonist (TC-5619) in negative and cognitive symptoms of schizophrenia. Schizophr Bull. (2016) 42:335–43. doi: x.1093/schbul/sbv072
PubMed Abstract | CrossRef Full Text | Google Scholar
41. Zhang XY, Liu L, Liu S, Hong X, Chen DC, Xiu MH, et al. Short-term tropisetron treatment and cognitive and P50 auditory gating deficits in schizophrenia. Am J Psychiatry. (2012) 169:974–81. doi: 10.1176/appi.ajp.2012.11081289
PubMed Abstruse | CrossRef Full Text | Google Scholar
42. Alpha LD, Summerfelt A, Lann H, Muller RJ. The negative symptom cess: a new instrument to assess negative symptoms of schizophrenia. Psychopharmacol Bull. (1989) 25:159–63.
PubMed Abstract | Google Scholar
43. Kay SR, Opler LA, Lindenmayer JP. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res. (1988) 23:99–110. doi: 10.1016/0165-1781(88)90038-8
PubMed Abstract | CrossRef Full Text | Google Scholar
44. Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry. (1989) 155:49–52. doi: 10.1192/S0007125000291496
PubMed Abstract | CrossRef Total Text | Google Scholar
45. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale: NJ: Lawrence Erlbaum Assembly. (1988)
Google Scholar
46. Higgins JPT, Dark-green South. Cochrane Handbook for Systematic Reviews of Interventions Version 5.i.0 [updated March 2011]. London: The Cochrane Collaboration. (2011)
Google Scholar
47. Hedges LV, Vevea JL. Fixed-and random-effects models in meta-analysis. Psychol Methods. (1998) iii:486. doi: x.1037/1082-989X.3.4.486
CrossRef Full Text | Google Scholar
48. Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package (2010). 36. doi: 10.18637/jss.v036.i03
CrossRef Full Text
50. Schünemann H, Brozek J, Oxman A, editors. Form handbook for grading quality of evidence and strength of recommendation. Version 3.2 [updated March 2009]. The GRADE Working Group. Bachelor online at: http://www.cc-ims.cyberspace/gradepro; 2009 (accessed July 6, 2020).
Google Scholar
51. Noroozian M, Ghasemi S, Hosseini SM, Modabbernia A, Khodaie-Ardakani MR, Mirshafiee O, et al. A placebo-controlled report of tropisetron added to risperidone for the treatment of negative symptoms in chronic and stable schizophrenia. Psychopharmacology. (2013) 228:595–602. doi: 10.1007/s00213-013-3064-2
PubMed Abstract | CrossRef Total Text | Google Scholar
52. Ghajar A, Gholamian F, Tabatabai-Motlagh Thousand, Afarideh M, Rezaei F, Ghazizadeh-Hashemi M, et al. Citicoline (CDP-choline) addition therapy to risperidone for treatment of negative symptoms in patients with stable schizophrenia: a double-blind, randomised placebo-controlled trial. Hum Psychopharmacol. (2018) 33:e2662. doi: 10.1002/hup.2662
PubMed Abstract | CrossRef Total Text | Google Scholar
53. Jin Y, Wang Q, Wang Y, Liu 1000, Sun A, Geng Z, et al. Alpha7 nAChR agonists for cerebral deficit and negative symptoms in schizophrenia: a meta-analysis of randomised double-blind controlled trials. Shanghai Arch Psychiatry. (2017) 29:191–9. doi: x.11919/j.issn.1002-0829.217044
PubMed Abstract | CrossRef Full Text | Google Scholar
54. Light-green MF, Nuechterlein KH, Gilded JM, Barch DM, Cohen J, Essock Due south, et al. Approaching a consensus cognitive bombardment for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and exam criteria. Biol Psychiatry. (2004) 56:301–vii. doi: 10.1016/j.biopsych.2004.06.023
PubMed Abstract | CrossRef Full Text | Google Scholar
56. Buchanan RW, Keefe RS, Umbricht D, Dark-green MF, Laughren T, Marder SR. The FDA-NIMH-MATRICS guidelines for clinical trial design of cognitive-enhancing drugs: what exercise we know 5 years subsequently? Schizophr Balderdash. (2011) 37:1209–17. doi: 10.1093/schbul/sbq038
PubMed Abstract | CrossRef Full Text | Google Scholar
57. Fusar-Poli P, Dette Yard, Smieskova R, Barbati S, Yung AR, Howes O, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. (2012) 69:562–71. doi: 10.1001/archgenpsychiatry.2011.1592
PubMed Abstruse | CrossRef Full Text | Google Scholar
58. Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high hazard to psychosis and first-episode psychosis: practise the cerebral deficits progress over, or after, the onset of psychosis? Schizophr Balderdash. (2014) twoscore:744–55. doi: ten.1093/schbul/sbt085
PubMed Abstruse | CrossRef Full Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.631589/full
0 Response to "Review of Alpha 7 Nachr Clinical Trials Schizophrenia"
Post a Comment